UroDapter®演示視頻
VIDEO PRESENTATION
Dr. Sándor Lovász on the use of UroDapter®
IBSA VIDEO TUTORIAL
Using the iAluAdapter® (UroDapter®)
UroDapter®是什么?
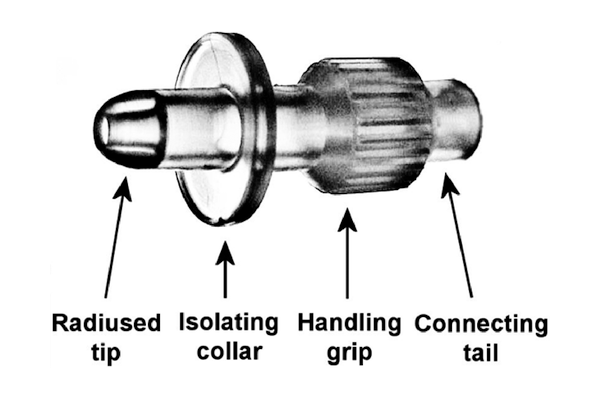
- 由医疗级弹性聚合物组成
- 特殊设计的圆角尖端可轻松触及外部尿道口
- 密封圈可确保无泄漏地注入膀胱
- 带有罗纹手柄,可在安装时快速固定
- 有连接尾巴可连接到卢尔接头和卢尔锁注射器
- 以6-8毫米的深度进入尿道
具有许多优点的小型设备
所有患者均首选UroDapter®而不选导管,因为:
- 使用UroDapter®进行滴注使用UroDapter®无痛
- UroDapter®不会对尿道造成损害
- 与导管不同,UroDapter®不增加尿路感染的风险
- 滴注引起的治疗后并发症少得多
- 使用UroDapter®,可以同时治疗膀胱和尿道
- 治疗的持续时间和费用都大大降低。
多种适应症的设备
溶液对附近的组织或器官没有不良影响的话,则可以将其与UroDapter®一起滴入膀胱。该设备可用于以下情况的治疗:
- 间质性膀胱炎/膀胱疼痛综合症(IC/BPS)
- 复发的尿路感染(UTIs)
- 癌症后治疗:化疗引起的膀胱炎
- 癌症后治疗:膀胱炎
- 女性患者膀胱癌症的复发预防
- 严重膀胱过度活动症(OAB)
- 针对任何适应症滴注止痛药,局部麻醉和消炎药
- UroDapter®也可以用于诊断目,例如: 逆行尿道造影,瘘管造影
强大法律背景
UroDapter®是一级的医疗设备。具有所需要的欧洲CE认证,也在美国FDA登记,在许多国家拥有强大的IP保护。
UroDapter®的专利正在申请中。
PCT国际申请编号:PCT/HU2016/000063
UroDapter®的销售信息
我们与IBSA达成了协议,将iAluadapter®/UroDapter®的营销和销售的专有权授予给他们。UroDapter®在以下国家,和 iAluRil®装在一个盒子里:阿尔巴尼亚,奥地利,白俄罗斯,比利时,波斯尼亚,保加利亚,克罗地亚,捷克共和国,塞浦路斯,丹麦,爱沙尼亚,芬兰,法国,德国,科索沃,希腊,匈牙利,爱尔兰,意大利,拉脱维亚,立陶宛,卢森堡,马其顿,马耳他, 荷兰,波兰,葡萄牙,罗马尼亚,塞尔维亚,斯洛伐克,斯洛文尼亚,西班牙,瑞典,英国,土耳其,澳大利亚和新西兰。
IBSA有权递送拥有iAluadapter®/UroDapter®的iAluRil®包装或者基于非排他性,适配器作为独立产品也可以在以下国家销售:乌克兰,俄罗斯,巴林,阿曼,科威特,卡塔尔,沙特阿拉伯,阿联酋,埃及,阿尔及利亚,约旦,巴勒斯坦,黎巴嫩,伊拉克,利比亚,摩洛哥,突尼斯,以色列,伊朗,韩国,印度尼西亚,中国,新加坡 ,台湾,土库曼斯坦,马来西亚,哥伦比亚,阿根廷,巴巴多斯,玻利维亚,巴西,智利,哥斯达黎加,多米尼加共和国,厄瓜多尔,萨尔瓦多,危地马拉,洪都拉斯,墨西哥,尼加拉瓜,巴拿马,巴拉圭,秘鲁,委内瑞拉,尼日利亚,肯尼亚, 加蓬和加纳。
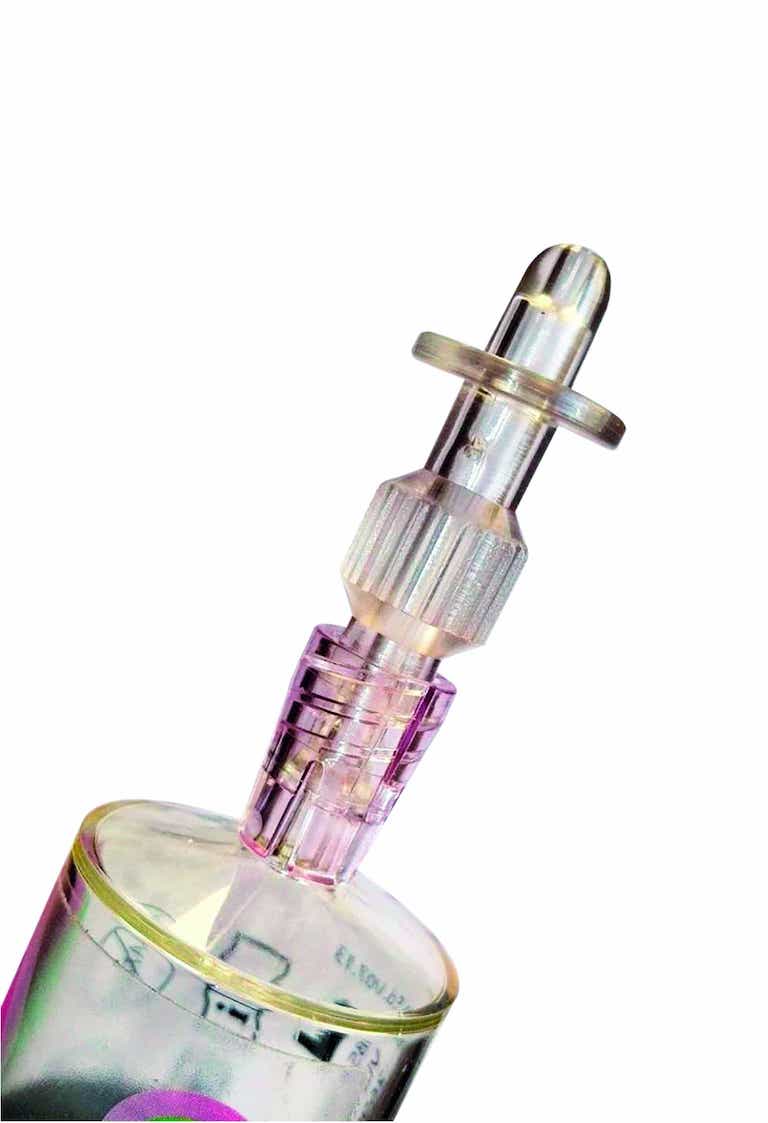
iAluadapter® (UroDapter®)
UroDapter® 可以直接连接到注射器上,以便将任何溶液滴入膀胱中
信息下载和链接
- Published Scientific Article of Sándor Lovász PhD, MD about UroDapter
- UroDapter®用户手册
- UroDapter® Tips & Tricks
- UroStill® & UroDapter® Flyer
- IBSA iAluadapter®/UroDapter® user manual
- IBSA iAluadapter®/UroDapter® user manual – female
- IBSA iAluadapter®/UroDapter® user manual – male
- iAluAdapter® tip sheet for patients
- iAluRil may be self-administered following appropriate training
- iAluAdapter® in Practice
- Catheter-free Instillation