UroDapter® presentation videos
VIDEO PRESENTATION
Dr. Sándor Lovász on the use of UroDapter®
IBSA VIDEO TUTORIAL
Using the iAluAdapter® (UroDapter®)
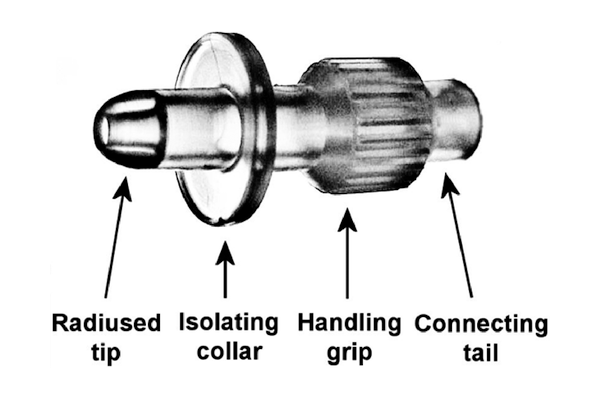
What is UroDapter®?
- Moulded from medical grade elastic polymer
- The specially designed radiused tip easily accesses the external urethral orifice
- The sealing collar enables the leakage-free instillation of the bladder
- With the ribbed grip it can be held fast when it is being mounted
- With the connecting tail it can be attached to both Luer Slip and Luer Lock syringes
- It enters the urethra merely in 6-8 mm depth
A small device with plenty of benefits
UroDapter® is preferred by the patients to catheters, because:
- Performing the instillation with UroDapter® is pain-free
- UroDapter®causes no lesions to the urethra
- Unlike catheters, UroDapter® does not raise the risk of urinary tract infections
- The instillation causes much less post treatment complications
- With UroDapter®, it is possible to treat the bladder and the urethra at the same time, which is impossible with a catheter
- Both the duration and the cost of the treatment is significantly lower
One device for many indications
Any solution can be instilled with UroDapter® into the bladder, assuming it has no adverse effect on the nearby tissues or organs. The device can be applied in the therapy of the following conditions:
- Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS)
- Recurring Urinary Tract Infections (UTIs)
- Post-cancer treatment: Chemotherapy-Induced Cystitis
- Post-cancer treatment: Radiation Cystitis
- Local recurrence prevention of Bladder Cancer, female patients
- Instilling analgesics, local anaesthetics, and antiphlogistics for any indication
- UroDapter® might be applied for diagnostic purposes, too – e.g. retrograde urethrography, fistulography
Strong legal background
- It has got the necessary European CE certificate, it is registered by the USA Food and Drug Administration (FDA), and has strong IP protection in many countries.
- UroDapter®'s patent is pending.
- PCT international patent application number: PCT/HU2016/000063
UroDapter® on the European market: iAluadapter®
We concluded an agreement with IBSA granting them exclusivity for the marketing and sale of iAluadapter®/UroDapter®, which is packed in one box with their product iAluRil® for the following countries: Albania, Austria, Belarus, Belgium, Bosnia, Bulgaria, Croatia, Czech Republic, Cyprus, Denmark, Estonia, Finland, France, Germany, Kosovo, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, North Macedonia, Malta, The Netherlands, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, The United Kingdom, Turkey, Australia, New Zealand.
IBSA has the right to deliver iAluRil® packages with iAluadapter®/UroDapter® and/or the adapter as a stand-alone product on the nonexclusive basis in the following countries: Ukraine, Russia, Bahrein, Oman, Kuwait, Qatar, Saudi Arabia, The United Arab Emirates, Egypt, Algeria, Jordan, Palestine, Lebanon, Iraq, Libya, Morocco, Tunisia, Israel, Iran, South Korea, Indonesia, China, Singapore, Taiwan, Turkmenistan, Malaysia, Colombia, Argentina, Barbados, Bolivia, Brazil, Chile, Costa Rica, Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Venezuela, Nigeria, Kenya, Gabon and Ghana.
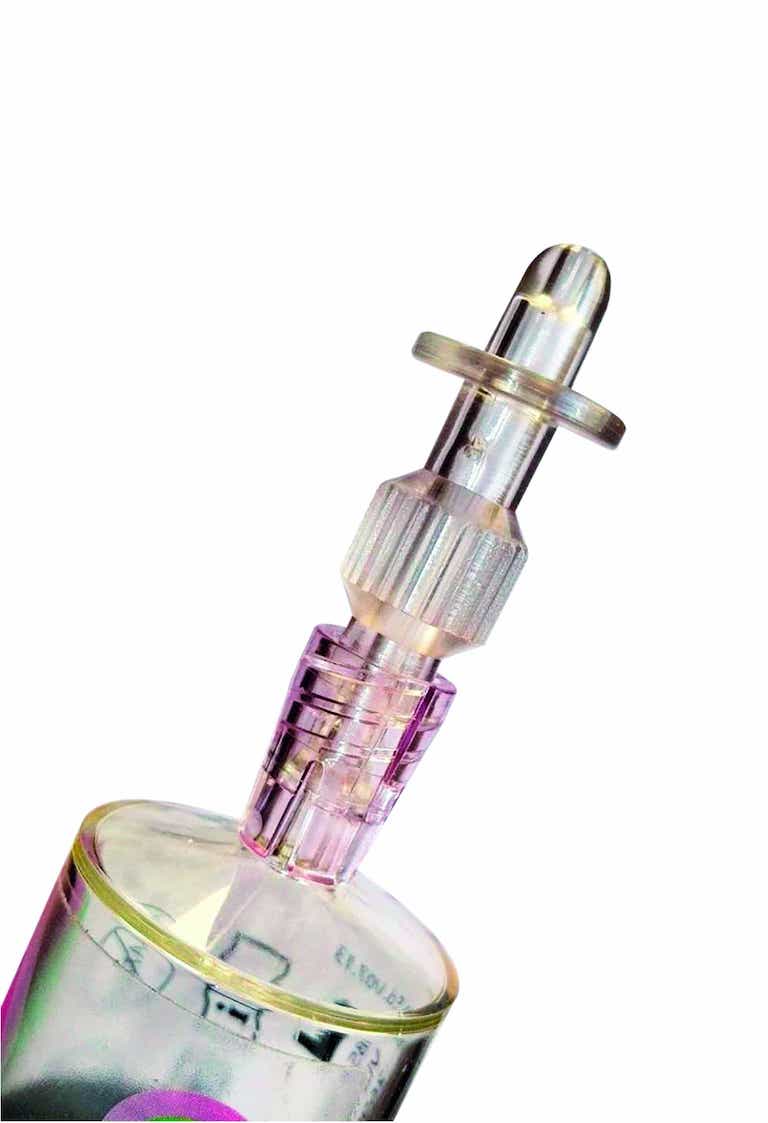
iAluadapter® (UroDapter®)
UroDapter® can be attached directly to the syringe so that any solution can be instilled into the bladder.
Information downloads & links
- International Journal of Urology (2019) 26 (Suppl. 1), 57–60
- UroDapter® user guide
- UroDapter® Tips & Tricks
- UroStill® & UroDapter® Flyer
- IBSA iAluadapter®/UroDapter® user manual
- IBSA iAluadapter®/UroDapter® user manual – female
- IBSA iAluadapter®/UroDapter® user manual – male
- iAluAdapter® tip sheet for patients
- iAluRil may be self-administered following appropriate training
- iAluAdapter® in Practice
- Catheter-free Instillation